A new battery chemistry could improve the efficiency and longevity of lithium-air batteries in a way that is “very scalable, cheap and much safer” than its predecessors, according to a paper published this week in Nature Energy.
Lithium-air batteries have the potential to deliver a high energy output relative to weight. But, previous designs have faced significant challenges including high energy losses, quick degradation, and expensive components that make them incompatible with conventional sealed batteries.
According to MIT Professor Ju Li and his co-authors in their new paper, their new nanolithia cathode battery design can overcome all of these challenges.
Battery technology is notoriously hard to improve. Nowhere was this more apparent than at the recent opening of Tesla’s Gigafactory, a massive battery plant in Nevada. According to its boss Elon Musk, Tesla built the factory because wringing more efficiency out of batteries is far more difficult than optimising the process by which they are made.
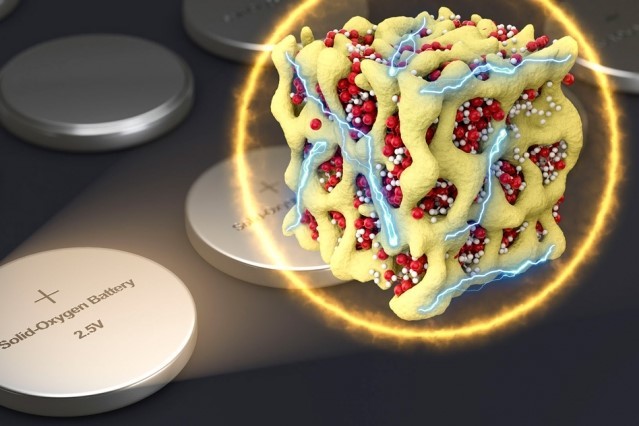
This image shows nanometer-scale particles made of lithium and oxygen compounds (depicted in red and white) which are embedded in a sponge-like lattice (yellow) of cobalt oxide, which keeps them stable, overcoming some existing issues in lithium-air battery designs. (Image courtesy of MIT.)
It is an ironic coincidence, that last month also saw the publication, in Nature Energy, of a paper outlining a way of making a battery whose prototype stores twice as much juice as the lithium-ion cells the Gigafactory will turn out, and which could eventually do better than that. The new battery, brainchild of Ju Li of the Massachusetts Institute of Technology, is some way from commercialisation, but its design is such that commercialising it should not be hard.
The fundamental idea behind Dr Li’s device is not new. It is a version of what is known as a lithium-air battery, something that has been a desideratum of energy-storage research since the 1970s. In theory, such batteries could hold more than four times the energy per kilogram of lithium-ion batteries. Building them, though, has proved taxing. As their name suggests, they draw in air. The part they need is the oxygen, but other atmospheric components—water vapour and carbon dioxide in particular—often damage them.
Even versions that run on pure oxygen, however, have been plagued with problems. Using and recharging existing lithium-air batteries wastes a huge amount of energy because the process involves changing the oxygen from a gaseous state into what is, in essence, a solid, and then back again. Such phase changes require a lot of energy and may thus waste more than 30% of the input electricity. Moreover, the changes in volume that accompany the shift from gas to solid to gas put a strain on the battery’s electrodes. This means they rapidly degenerate to the point where the battery can no longer be recharged.
The crucial difference between Dr Li’s design and previous attempts is that no actual air is involved. Instead, the cell is hermetically sealed and uses oxygen stored inside the battery itself, in a chemical called lithium superoxide (LiO2). Because this compound is unstable, it is easily induced to surrender some of its oxygen.
To stop the superoxide disintegrating spontaneously, Dr Li embed it in the voids of a matrix made of cobalt oxide. This gives the superoxide’s structure stability.
When the new battery is discharging power, lithium ions from a liquid electrolyte that bathes the matrix enter the solid and react with the oxygen in the superoxide to form either lithium peroxide (Li2O2) or lithium oxide (Li2O), both of which are also solids. Those chemical reactions drive electrons around an external circuit, where they might be put to use running anything from a mobile phone to a vehicle’s electric motor. Push electrons the other way around the circuit, though, by connecting the battery to a power supply, and the chemical reactions will go into reverse, charging the thing up again.
That the oxygen remains in a solid state throughout these processes is crucial to the new battery’s success. Instead of 30%, it loses just 8% of the energy put into it. Similarly, its life is prolonged. In trials which discharged and recharged the battery 130 times, it lost less than 2% of its capacity.
Past claims of practical lithium-air batteries have been met with scepticism, but in this case other workers in the field who are not involved in the study seem persuaded that Dr Li may be onto something. “Really impressive,” says Venkat Viswanathan of Carnegie Mellon University, in Pittsburgh. “A very interesting, exciting piece of work,” agrees Laurence Hardwick of Liverpool University, in Britain.
Dr Li hopes, within a year, to turn the prototype into something that might be manufactured. This is an ambitious goal but Dr Hardwick agrees that, from an engineering perspective, the challenges are similar to conventional lithium-ion batteries, so rapid development is possible. And it is also an attractive goal. For Tesla and its rivals, these batteries could fuel a virtuous cycle of lighter cars with longer ranges. Dr Li sees this potential, too. His team have filed a patent and have begun talking with manufacturers.
Sources: Economist and Scientific America
